Understanding Halogen Bonding in Solution
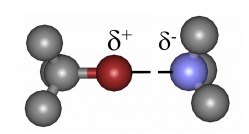
Halogen bonding is an electron density donation-based weak interaction that has so far mostly been investigated in computational and crystallographic studies.
We examine halogen bonding formed in solution environment employing a novel, exceedingly accurate NMR methodology. Using a combination of spectroscopic and computational techniques the energetics of halogen bonds and the role of electrostatic and non-electrostatic effects in the interaction will be studied. Variation of bond length and symmetry as well as their relevance in inter- and intramolecular interactions are being elucidated.
The gained knowledge will be applied to understand the impact of halogen bonding in biological systems, such as protein-ligand and lipide-ligand interactions as well as used to develop new applications of halogen bonding in organic, analytical and pharmaceutical chemistry.
Our work is financially supported by the Swedish Scientific Research Council, the European Research Council, the Carl Tryggers Foundation, the Åke Wibergs Foundation, the Royal Society of Arts and Sciences in Göteborg, and the Swedish Foundation for Internationalization in Research and Higher Education.
Some recent publications:
Halogen bond activation in gold catalysis
Freyer Jónsson, H.; Sethio, D.; Wolf, J.; Huber, S.M.; Fiksdahl, A.; Erdelyi, M.
ACS Catalysis 2022, 12, 7210-7220
Pushing the limits of characterising a weak halogen bond in solution
Peintner, S.; Erdelyi, M.
Chem. Eur J. 2022, 28, e202103559.
The influence of secondary interactions on the [N-I-N]+ halogen bond
Lindblad, S.; Boróka Németh, F.; Földes, T.; von der Heiden, D.; Vang, H.G.; Driscoll, Z.L.; Gonnering, E.R.; Pápai, I.; Bowling, N.; Erdelyi, M.
Chem. Eur. J. 2021, 27, 13748-13756
Probing halogen bonds by scalar couplings
Jimmink. B.; Sethio, D.; Turunen, L.; von der Heiden, D.; Erdelyi, M.
J. Am. Chem. Soc. 2021,143, 10695-10699
Halogen Bonds of Halonium Ions
Turunen, L; Erdelyi, M.
Chem. Soc. Rev. 2020, 49, 2688-2700
Natural Product Isolation from Medicinal Plant of Eastern African Countries
We have ongoing collaborations with the groups of Prof. Abiy Yenesew (University of Nairobi, Kenya), Dr. Stephen Nyandoro (University of Dar es Salaam) Prof. Théoneste Muhizi and Dr. Daniel Umereweneza (University of Rwanda) and Ahmed Mohammed (Cape Peninsula University of Technology, South Africa) and aim to join our forces by integrating cultural practice with modern pharmaceutical and chemical approaches. Expected outcomes of the projects are bioactive natural products rapidly transferable into drugs to the treatment of a variety of diseases - either through identification of new lead compounds or through development of phytomedicines.
Some recent publication:
Crotofolane Diterpenoids and Other Constituents Isolated from Croton kilwae
Mahambo, E.T.; Uwamariya, C.; Miah, M.; da Costa Clementino, L.; Salazar Alvarez, L.C.; Di Santo Metzler, G. P.; Trybala, E.; Said, J.; Wieske, L.H.E.; Ward, J.; Rissanen, K.; Munissi, J.J.E.; Costa, F.T.M.; Sunnerhagen, P.; Bergström,T.; Nyandoro, S.S.; Erdelyi, M.
J. Nat. Prod. 2023, 86, 380-389
Macrocyclic Pyrrolizidine Alkaloids and Silphiperfolanol Angelate Esters from Solanecio mannii
Umereweneza, D.; Atilaw, Y.; Peintner, S.; Rudenko, A.; Bourgard, C.; Xiong, R.; Muhizi, T.; Sunnerhagen, P.; Gogoll, A.; Erdelyi, M.
Eur J. Org. Chem. 2023, e202201280
Modified ent-Abietane Diterpenoids from the Leaves of Suregada zanzibariensis
Kalenga, T.M.; Mollel, J.T.; Said, J.; Orthaber, A.; Ward, J.S.; Atilaw, Y.; Umereweneza, D.; Ndoile, M.M.; Munissi, J.J.E.; Rissanen, K.; Trybala, E.; Bergstrom, T.; Nyandoro, S. S.; Erdelyi, M.
J. Nat. Prod. 2022, 85, 2135-2141
A new β-hydroxydihydrochalcone from Tephrosia uniflora, and the revision of three β-hydroxydihydrochalcones to flavanones
Chepkirui, C.; Bourgard, C.; Gilissen, P.J.; Ndakala, A.; Derese, S.; Gütlin, Y.; Erdelyi, M.; Yenesew, A.
Fitoterapia 2022, 158, 105166
Antiviral iridoid glycosides from Clerodendrum myricoides
Umereweneza, D.; Molel, J.T.; Said, J.; Atilaw, Y.; Muhizi, T.; Trybala, E.; Bergström, T.; Gogoll, A.; Erdelyi, M.
Fitoterapia 2021, 155, 105055
Development of Metallo-beta-lactamase Inhibitors to Combat Antibiotic Resistance
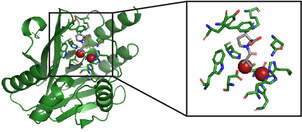
This project deals with different MβLs such as NDM-1, VIM-2 and CcrA. The project involves MβL expression and purification and development of potent broad-spectrum MβL inhibitors. The inhibitors are transition-state analogs of the beta-lactam antibiotic undergoing hydrolysis. Their broad-spectrum inhibition activity and structure-activity relationship is evaluated. Special attention is given to possible synergestic effects when applied in combination with antibiotics for inhibition of antibiotic resistant bacteria. The complexes of MβLs and the transition state analogs are studied by solution NMR and X-ray crystallography, to provide information useful for the development of clinically applicable broad spectrum inhibitors. The long term goal of this project is to create novel compounds with broad-spectrum inhibitory potency to MβL producing multi-drug resistant bacteria, and to provide a better understanding of the molecular processes behind antibiotic resistance.
This project is part of the Uppsala antibiotic centre (https://www.uac.uu.se/) and we collaborate with Hanna-Kirsti Leiros and Annette Bayer (UiT, Norway) as well as with Weiliang Zhu (Chinese Academy of Sciences, China) on this project.
Some recent publication:
NMR Backbone Assignment of VIM-2 and Identification of the Active Enantiomer of a Potential Inhibitor
Wieske, L.H.E., Bogaerts, J., Leding, A.A.M., Wilcox, S., Andersson Rasmussen, A., Leszczak, K., Turunen, L., Herrebout, W.E., Hubert, M., Bayer, A., Erdelyi, M.
ACS Med. Chem. Lett. 2022, 13, 257-261
Metallo-β-Lactamase Inhibitor Phosphonamidate Monoesters
Palica, K.; Vorácová, M.; Skagseth, S.; Andersson Rasmussen, A.; Allander, L.; Hubert, M.; Sandegren, L.; Schrøder Leiros, H. K.; Andersson, H. ; Erdelyi, M.
ACS Omega 2022, 7, 4550-4562
Binding of 2-(Triazolylthio)acetamides to Metallo-β-lactamase CcrA Determined with NMR
Andersson, H.; Jarvoll, P.; Yang, S.-K.; Yang, K.-W.; Erdelyi, M.
ACS Omega 2020, 5, 21570–21578
Obtaining Solution Structures
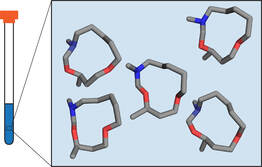
We have several collaboration projects where we determine solution ensembles of conformations to answer medicinal chemistry related questions our collaborators have. Some of our long-term collaborators are Prof. Jan Kihlberg (Uppsala university, Sweden), and Prof. Alethea Tabor (University college London, The United Kingdom).
Some recent publication:
Linker-Dependent Folding Rationalizes PROTAC Cell Permeability
Poongavanam, V.; Atilaw, Y.; Siegel., S.; Giese, A.; Lehmann, L.; Meibom, D.; Erdelyi, M.; Kihlberg, J.
J. Med. Chem. 2022, 65, 13029-13040
Cell Permeability of Isomeric Macrocycles: Predictions and NMR Studies
Begnini, F.; Poongavanam, V.; Atilaw, Y.; Erdelyi, M.; Schiesser, S.; Kihlberg, K.
ACS Med. Chem. Lett. 2021, 12, 983-990
Solution-State Preorganization of Cyclic Hairpin Ligands Determines Binding Mechanism and Affinities for MDM2
Ge, Y.; Zhang, S.; Erdelyi, M.; Voelz, V. A.
J. Chem. Inf. Model. 2021, 61, 2353-2367
Employing complementary spectroscopies to study the conformations of an epimeric pair of side-chain stapled peptides in aqueous solution
Bogaerts, J.; Atilaw, Y.; Peintner. S.; Aerts, R.; Kihlberg, J.; Johannessen, C.; Erdelyi, M.
RSC. Adv. 2021, 11, 4200-4208.
A Chemical Biology Approach to Under.standing Molecular Recognition of Lipid II by Nisin(1-12): Synthesis and NMR Ensemble Analysis of Nisin(1-12) and Analogues
Dickman, R.; Danelius, E.; Mitchell, S.; Hansen, F.; Erdelyi, M.; Tabor, A.B.
Chem. Eur. J. 2019, 25, 14572-14582.
